Technical Notes
Your knowledgebase for DAISOGEL silica gel products
-

Are DAISOGEL Products Registered in FDA DMF?
Read More >According to the US FDA, “drug master files (DMFs) are submissions to FDA used to provide confidential, detailed information about facilities, processes, or articles used in the manufacturing, processing, packaging, and storing of human drug products.” There are several types of DMFs. Type II DMFs are applied to “Drug Substance, Drug Substance Intermediate, and Material Used in Their Preparation; or Drug Product.” The top-selling DAISOGEL phases have Type II FDA DMF registration. Our rigid manufacturing standards guarantee that only the highest quality silica gel products are delivered to our valued customers. We have FDA DMF registration for the following phases:
-

What is the pH Range of DAISOGEL C8 and ODS Phases?
Read More >Are you purifying a compound that requires a very low pH condition for solubility or elution purposes? Perhaps you need a silica gel that can withstand caustic cleaning instead. The pH stability of a C8 or ODS silica gel is dependent on the robustness of the bare silica gel, as well as the ligand density and the quality of the bonding. DAISOGEL offers a wide range of bonded phases with various ligand densities to give you choices for finding the right match for your application. Your guide to the pH range of our most common C8 and ODS phases: Contact
-
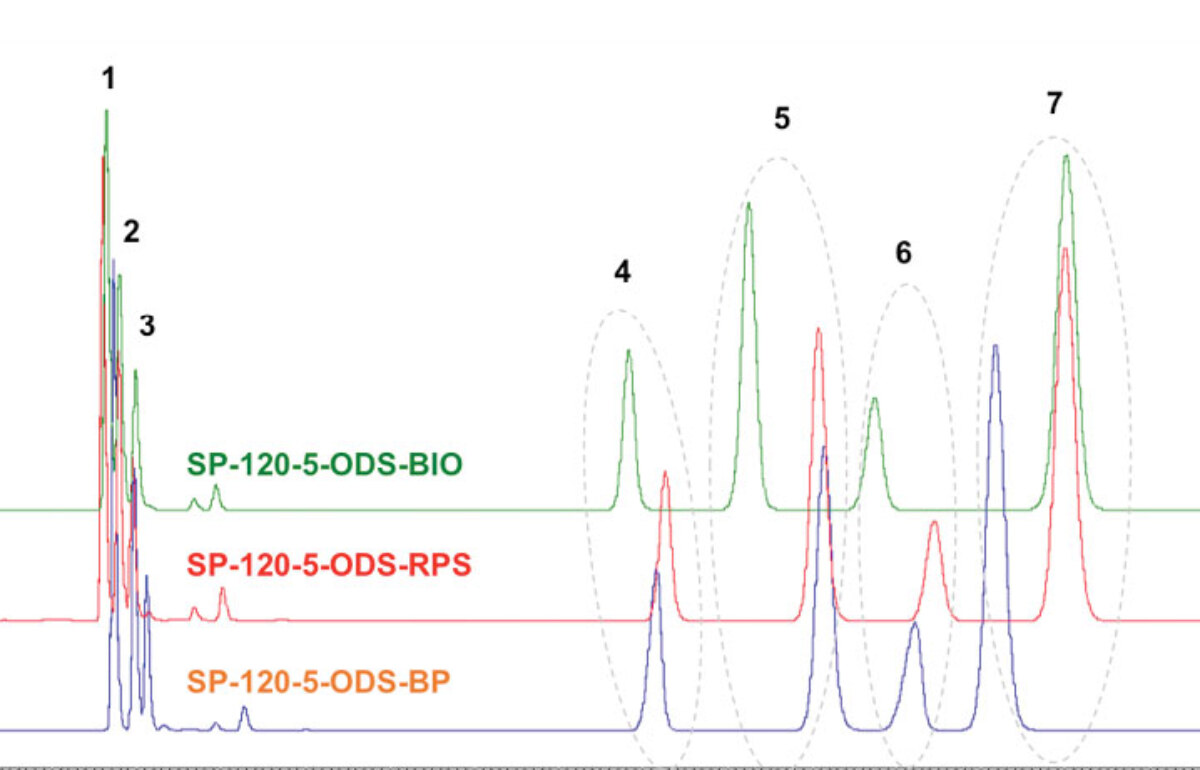
What is the Role of Ligand Density for Separation?
Read More >When meeting with potential bulk silica customers for the first time, we are often asked the following question: “Do you have an ODS/C18 phase?” While the answer is, of course, a resounding yes, we also suggest that this is not the right question to be asking your silica gel supplier! The right question is: “What different ligand densities do you offer for your ODS/C18 phases?” With ligand density (sometimes called bonding density), we express how much of the given ligand is grafted onto the silica’s surface – not only onto the outer particle surface, but also on the INSIDE of
-

Seven Differentiators of DAISOGEL
Read More >There are many suppliers from around the world that offer bulk silica gel for chromatography. This plethora of supply gives you choices…and choices are a good thing for the chromatographer. So what is it that makes DAISOGEL different? What sets DAISOGEL apart from the other bulk silica gels on the market? Manufacturing your API is cash-intensive, and no doubt your management must carefully monitor the cash flow of the business. Contact us to learn more Reach out to see how DAISOGEL can help you sustain your mission-critical purification process.
-
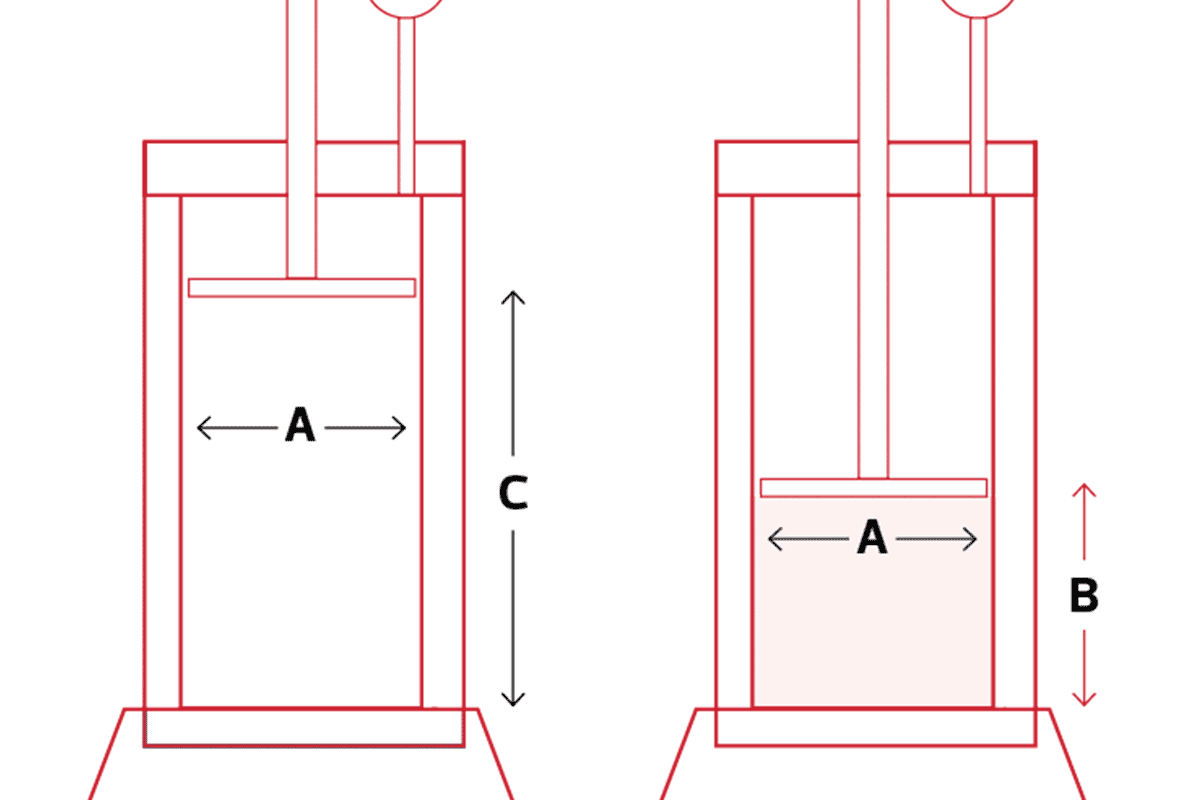
Packing and Unpacking DAISOGEL C8-PK Series Phase
Read More >Once you scale up your process beyond semi-prep or preparative prepacked columns, you will need to pack our bulk silica gel in a dynamic axial compression (DAC) column. Here, we provide a procedure for packing, testing, and unpacking one of our most prominent silica gels: the C8-PK Series with 10 µm particle size (Product Name SP-100-10-C8-PK). Calculate the Necessary Silica Gel and Volume Required The first step is to determine the amount of silica gel and slurry solvent required to pack your column. You can do that by using these simple calculations: Start with the dimensions of your empty column
-
Which DAISOGEL grade is best for small molecule purification?
Read More >The key for a good small molecule separation–or any chromatographic separation, for that matter–is that your target molecule has access to the entire surface area of the silica gel-based stationary phase. The pore size of the bead must be wide enough for this purpose, yet small enough for the silica product to have a large enough surface area to provide an effective loadability and ensure optimized process economics. In fact, the surface area and the pore size have direct correlation in DAISOGEL products. For small molecules, there is one grade in particular that strikes the right balance between pore size
-
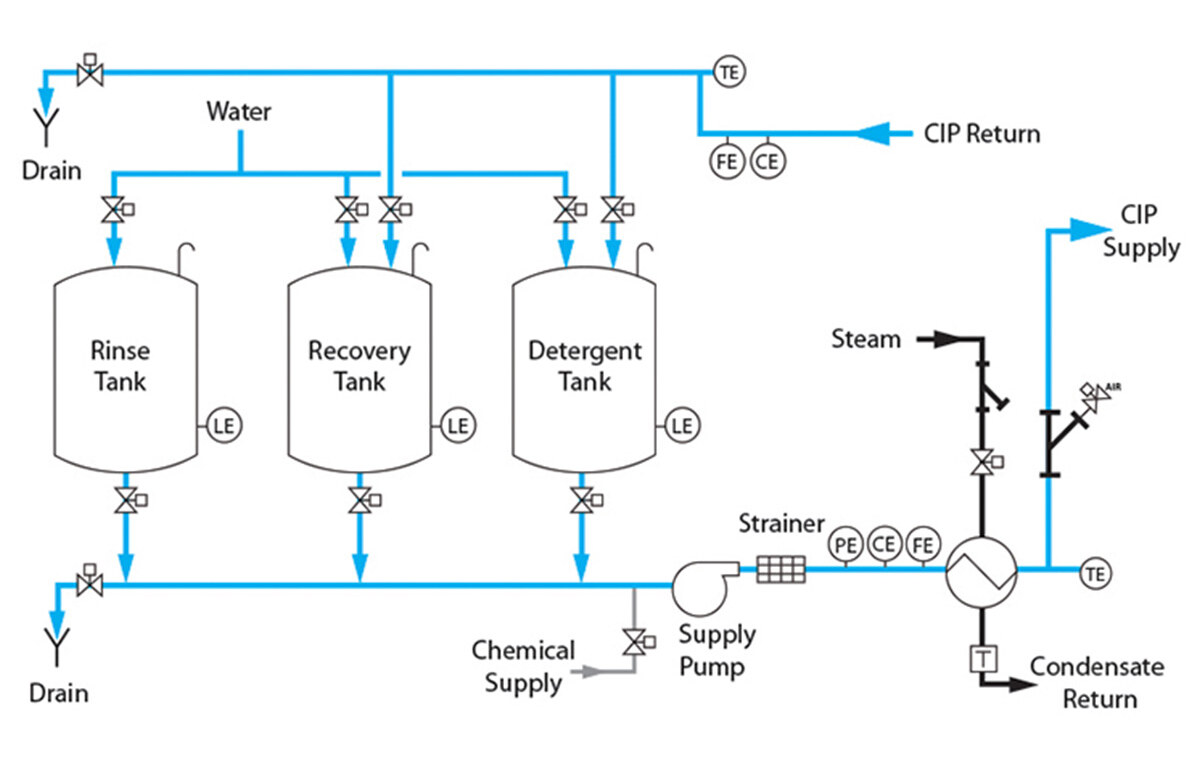
Considerations for Performing Cleaning-in-Place (CIP)
Read More >Manufacturing managers in charge of the downstream processing plant for synthetic or recombinant peptides know very well that CIP of a packed chromatography column is a “necessary evil” to extend the lifetime of the bed. While CIP is a step that one may want to avoid as much as possible, it is also an economically important practice that can reduce the cost of manufacturing your API. Considerations for performing CIP on columns packed with a bonded phase silica gel The first and primary consideration is the CIP agent–what chemical to use. Of course, a milder chemical with pH closer to
-

Column Regeneration Suggestions
Read More >Even well-packed columns will lose some of their efficiency over long usage periods of many cycles, especially when used with samples of high impurity level. If your column is used for synthetic peptide or recombinant peptide purification, or, in a worst-case, for aggregating, fibrillating peptide purification, the column performance can decrease rather quickly. The first warning sign is usually the rising backpressure of the column. Peak shape deterioration or the drop in the number of theoretical plates may be another sign that you need to clean off the fibrillated peptide building up on the inlet side of the silica bed
-

Mechanical Strength Differences Between Grades of DAISOGEL
Read More >The mechanical strength of a chromatographic silica gel product is basically determined by its manufacturing process. The SOL-GEL method we use is a sure ticket for the best quality products. That said, there are differences in mechanical strength of the products in our portfolio. In the wide DAISOGEL lineup we offer the Wide Pore and Super Wide Pore varieties. As you might imagine, the wider the pore diameter of silica gel, the gentler care the product needs. In that sense, wider pore silica must be packed at lower packing pressure to avoid damage to the particles. A similar concept holds
-
Particle Size Discrepancy Between Bulk Silica Gel Products
Read More >When you work in a purification process development laboratory, you undoubtedly want to screen several silica gel products from various manufacturers in order to determine which silica product has the best selectivity and overall yield for your given API. To design a robust screening study, the process development scientist knows to compare “apples to apples.” It might not be well-known, but there can be a difference in the particle size values published on Certificates of Analysis (so-called “nominal” size) versus actual particle size with some silica gel suppliers. Naturally, different particle size measurement methods will yield different results, and to
